 Get premium membership
Get premium membership and access questions with answers, video lessons as well as revision papers.
- Study the reduction potentials below.
(i) Identify the weakest oxidizing agent.
(ii) Calculate the e.m.f of the cell that would produce the highest output of voltage.
(iii)...(Solved)
Study the reduction potentials below.
 (i) Identify the weakest oxidizing agent.
(ii) Calculate the e.m.f of the cell that would produce the highest output of voltage.
(iii) Write the cell diagram for the cell formed above.
(i) Identify the weakest oxidizing agent.
(ii) Calculate the e.m.f of the cell that would produce the highest output of voltage.
(iii) Write the cell diagram for the cell formed above.
Date posted: May 17, 2019.
- Study the diagram below and answer the questions which follow.(Solved)
Study the diagram below and answer the questions which follow.
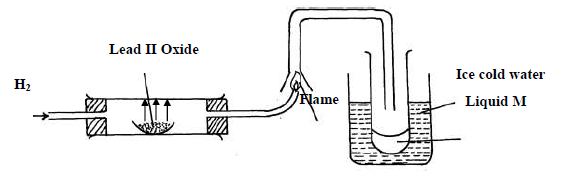 (i) State two observations made when hydrogen gas pass over hot lead II oxide.
(ii) Write the equation for the reaction which occurs in the combustion tube.
(iii) What property of hydrogen is shown in the experiment above
(iv) Identify liquid M.
(v) What type of reaction occurs when hydrogen gas reacts with butene?
(vi) State the condition required for the reaction (v) above
(vii) Apart from hydrogen peroxide, state two other reagents that can be used to prepare
oxygen gas.
(viii) Write an equation to show how hydrogen gas is formed from the reagents chosen in
(vii) above.
(i) State two observations made when hydrogen gas pass over hot lead II oxide.
(ii) Write the equation for the reaction which occurs in the combustion tube.
(iii) What property of hydrogen is shown in the experiment above
(iv) Identify liquid M.
(v) What type of reaction occurs when hydrogen gas reacts with butene?
(vi) State the condition required for the reaction (v) above
(vii) Apart from hydrogen peroxide, state two other reagents that can be used to prepare
oxygen gas.
(viii) Write an equation to show how hydrogen gas is formed from the reagents chosen in
(vii) above.
Date posted: May 17, 2019.
- What is atomicity?(Solved)
What is atomicity?
Date posted: May 17, 2019.
- Study the periodic grid below and answer the questions which follow. The letters do not
represent actual symbols of the elements.(Solved)
Study the periodic grid below and answer the questions which follow. The letters do not
represent actual symbols of the elements.
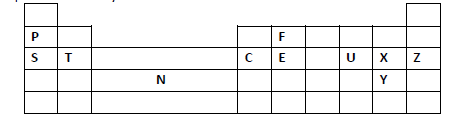 (i) To which category of elements does element N belong?
(ii) Compare the atomic radius of element U and X. Explain.
(iii) An ion A3- has a configuration of 2.8. Place element A on the grid above.
(iv) Which of the group 1 elements will require the greatest amount of energy to
remove the outermost electron. Explain.
(v) Why is element Z used in light bulbs?
(vi) Write the formula of the phosphate of element T.
(vii) State the type of structure found in the oxide of element F.
(i) To which category of elements does element N belong?
(ii) Compare the atomic radius of element U and X. Explain.
(iii) An ion A3- has a configuration of 2.8. Place element A on the grid above.
(iv) Which of the group 1 elements will require the greatest amount of energy to
remove the outermost electron. Explain.
(v) Why is element Z used in light bulbs?
(vi) Write the formula of the phosphate of element T.
(vii) State the type of structure found in the oxide of element F.
Date posted: May 17, 2019.
- A given amount of propane was used to heat one litre of water. The temperature of
the water rose from 25oC to 50.5oC. (S.H.C of water...(Solved)
A given amount of propane was used to heat one litre of water. The temperature of
the water rose from 25oC to 50.5oC. (S.H.C of water = 4.2J/g/k)
(i) Calculate the heat change for the reaction.
(ii) Find the mass of propane burnt (C=12, H=1)
Date posted: May 17, 2019.
- Study the heats of combustion shown below.(Solved)
Study the heats of combustion shown below.
 Draw an energy cycle diagram linking heat of formation of propane with its heat of
combustion and the heat of combustion of the constituent elements.
Draw an energy cycle diagram linking heat of formation of propane with its heat of
combustion and the heat of combustion of the constituent elements.
Date posted: May 17, 2019.
- Define standard heat of combustion of a substance.(Solved)
Define standard heat of combustion of a substance.
Date posted: May 17, 2019.
- Study the diagram below. State and explain the observation made after sometime(Solved)
Study the diagram below.
 State and explain the observation made after sometime.
State and explain the observation made after sometime.
Date posted: May 17, 2019.
- Give an equation to show how chlorine forms bleaching powder.(Solved)
Give an equation to show how chlorine forms bleaching powder.
Date posted: May 17, 2019.
- Chlorine reacts with cold dilute sodium hydroxide to form a bleaching agent.
Name the bleaching agent.(Solved)
Chlorine reacts with cold dilute sodium hydroxide to form a bleaching agent.
Name the bleaching agent.
Date posted: May 17, 2019.
- The set up below was used to prepare chlorine gas. (i) Identify solid M (ii) What is the role of water in the experiment?.....(Solved)
The set up below was used to prepare chlorine gas.
 (i) Identify solid M
(ii) What is the role of water in the experiment?
(iii) Complete the set up to show how dry chlorine gas can be collected
(iv) Write a chemical equation to show how chlorine gas is formed.
(i) Identify solid M
(ii) What is the role of water in the experiment?
(iii) Complete the set up to show how dry chlorine gas can be collected
(iv) Write a chemical equation to show how chlorine gas is formed.
Date posted: May 17, 2019.
- State one use of lead.(Solved)
State one use of lead.
Date posted: May 17, 2019.
- Pure lead can be obtained by electrolysis. Identify the anode and cathode for the
process.(Solved)
Pure lead can be obtained by electrolysis. Identify the anode and cathode for the
process.
Date posted: May 17, 2019.
- The flow chart below summarizes the process of extraction of lead from a chief ore.(Solved)
The flow chart below summarizes the process of extraction of lead from a chief ore.
 (i) Identify process T
(ii) Give the name of:
Gas N
Solid F
(iii) Give two functions of CaCO3 in the extraction process.
(iv) Write an equation to show how waste product J is formed.
(i) Identify process T
(ii) Give the name of:
Gas N
Solid F
(iii) Give two functions of CaCO3 in the extraction process.
(iv) Write an equation to show how waste product J is formed.
Date posted: May 17, 2019.
- Name any two ores of lead.(Solved)
Name any two ores of lead.
Date posted: May 17, 2019.
- a)Write down the electron arrangement for an atom of element U which has a mass number 14 and
contains 8 neutrons.(Solved)
a)Write down the electron arrangement for an atom of element U which has a mass number 14 and
contains 8 neutrons.
(b) Draw the structure of an atom of A given in (a) above.
Date posted: May 17, 2019.
- A sealed glass tube containing 250 cm3 of nitrogen gas at r.t.p was immersed in boiling water. Calculate the pressure inside the tube if the...(Solved)
A sealed glass tube containing 250 cm3 of nitrogen gas at r.t.p was immersed in boiling water. Calculate the pressure inside the tube if the volume of the gas does not change due to expansion of glass. (Room pressure=760mmHg, room temperature=298K).
Date posted: May 17, 2019.
- Explain this observation:
When hydrogen chloride gas is dissolved in water, the solution conducts electricity while a
solution of hydrogen chloride gas in propanone does not conduct...(Solved)
Explain this observation:
When hydrogen chloride gas is dissolved in water, the solution conducts electricity while a
solution of hydrogen chloride gas in propanone does not conduct electricity
Date posted: May 17, 2019.
- A solution of bromine in methyl benzene turns colourless when butane gas is passed through it.(Solved)
A solution of bromine in methyl benzene turns colourless when butane gas is passed through it.
(a) What type of reaction takes place?
(b) Write equation of the reaction which takes place.
Date posted: May 17, 2019.
- The thermochemical equation below shows a dynamic equilibrium between hydrogen iodide
gas and its elements:(Solved)
The thermochemical equation below shows a dynamic equilibrium between hydrogen iodide
gas and its elements:
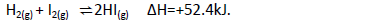 (a) Explain how the following changes would affect the production of hydrogen iodide.
(i) increase in temperature
(ii) decrease in pressure.
(b) Calculate the molar enthalpy for formation of HI (g).
(a) Explain how the following changes would affect the production of hydrogen iodide.
(i) increase in temperature
(ii) decrease in pressure.
(b) Calculate the molar enthalpy for formation of HI (g).
Date posted: May 17, 2019.
- 60 cm3 of ozone (O3) diffused through a semi permeable membrane in 80 seconds. Calculate the time taken for 90 cm3 of nitrogen (IV) oxide...(Solved)
60 cm3 of ozone (O3) diffused through a semi permeable membrane in 80 seconds. Calculate the time taken for 90 cm3 of nitrogen (IV) oxide (NO2) to diffuse under the same conditions. (O=16,N=14).
Date posted: May 17, 2019.
- When a current of 2.0 amperes was passed through a cell containing aqueous solution of T2+
ions of metal T for 9 minutes the mass of...(Solved)
When a current of 2.0 amperes was passed through a cell containing aqueous solution of T2+
ions of metal T for 9 minutes the mass of the cathode increased by 0.36g.(1Faraday=96,500coulombs)
(a) Calculate the quantity of electricity used.
(b) Determine the relative atomic mass of metal T.
(c) Explain whether metal T is less or more reactive than hydrogen gas.
Date posted: May 17, 2019.
- In an experiment 1.2g of granulated zinc were reacted with excess 2.0M sulphuric acid. The
time taken for the reaction to be completed was recorded. The...(Solved)
In an experiment 1.2g of granulated zinc were reacted with excess 2.0M sulphuric acid. The
time taken for the reaction to be completed was recorded. The experiment was repeated using
1.2g of zinc powder.
(a) In which experiment was the time taken shorter? Explain your answer.
(b) The mass of remaining mass of zinc was measured as time moved until when the
reaction was over. Sketch on the set of axes and label the two curves obtained that
would represent the mass of the remaining zinc
Experiment I represents granulated zinc.
Experiment II represents zinc powder.

Date posted: May 17, 2019.
- The set up below was used by a student. Filter paper soaked in purple litmus solution was
placed in the middle of the combustion tube.(Solved)
The set up below was used by a student. Filter paper soaked in purple litmus solution was
placed in the middle of the combustion tube.
 (i) What is the main aim of the experiment.
(ii) State the first observation likely to have been made in the tube. Explain the observation.
(i) What is the main aim of the experiment.
(ii) State the first observation likely to have been made in the tube. Explain the observation.
Date posted: May 17, 2019.
- 0.5g of Manganese (IV) oxide were added to 50 cm3 of 3.5M hydrogen peroxide. The
temperature of the solution rose from 21oC to 64oC. The information...(Solved)
0.5g of Manganese (IV) oxide were added to 50 cm3 of 3.5M hydrogen peroxide. The
temperature of the solution rose from 21oC to 64oC. The information was represented on an energy level diagram as shown.
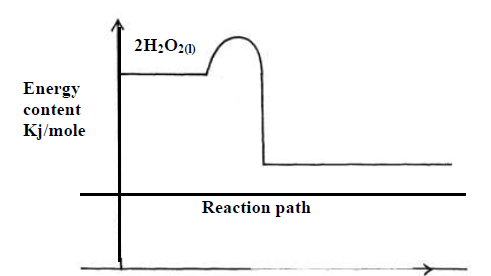 (a) Determine the number of moles of hydrogen peroxide that decomposed.
(b) Calculate the molar enthalpy of decomposition of hydrogen peroxide.
(c) On the same set of axes above sketch the curve that would be obtained if manganese (IV)
oxide was not used and other conditions remained constant.
(a) Determine the number of moles of hydrogen peroxide that decomposed.
(b) Calculate the molar enthalpy of decomposition of hydrogen peroxide.
(c) On the same set of axes above sketch the curve that would be obtained if manganese (IV)
oxide was not used and other conditions remained constant.
Date posted: May 17, 2019.
- In a reaction, an alkanol B was converted to pent-2-ene(Solved)
In a reaction, an alkanol B was converted to pent-2-ene
(a) Give the structural formula of alkanol B.
(b) Name (i) the type of reaction that converts alkanol B to pent-2-ene.
(ii) The reagent used.
Date posted: May 17, 2019.
- The diagram below is a set up used to investigate the effect of heat on hydrated copper(II)
sulphate. Study the diagram and answer the questions that...(Solved)
The diagram below is a set up used to investigate the effect of heat on hydrated copper(II)
sulphate. Study the diagram and answer the questions that follow.
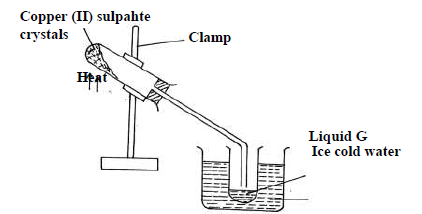 (a) Why is boiling tube slanted as shown?
(b) What is observed in the boiling tube.
(c) Identify liquid G.
(a) Why is boiling tube slanted as shown?
(b) What is observed in the boiling tube.
(c) Identify liquid G.
Date posted: May 17, 2019.
- Explain why the following combination of reagents is unsuitable for the laboratory
preparation of hydrogen.(Solved)
Explain why the following combination of reagents is unsuitable for the laboratory
preparation of hydrogen.
(i) Zinc + dilute nitric acid.
(ii) Lead + dilute hydrochloric acid.
(iii) Potassium + dilute sulphuric acid.
Date posted: May 17, 2019.
- Give names of the following processes used to:
(Solved)
Give names of the following processes used to:
(a) Obtain a solvent from a saturated solution.
(b) Remove steam from air
(c) Separate zinc carbonate from water
(d) Separate a mixture of nitrogen and helium.
Date posted: May 17, 2019.
- Describe a simple test that can be carried out in the laboratory to distinguish between manganese (IV) oxide and copper (II) oxide.(Solved)
Describe a simple test that can be carried out in the laboratory to distinguish between manganese (IV) oxide and copper (II) oxide.
Date posted: May 17, 2019.