 Get premium membership
Get premium membership and access questions with answers, video lessons as well as revision papers.
- State three properties common to both ammonia and calcium hydroxide solutions but different
from solution of sulphur (IV) oxide in water.(Solved)
State three properties common to both ammonia and calcium hydroxide solutions but different
from solution of sulphur (IV) oxide in water.
Date posted: May 17, 2019.
- A current of 25 amps was passed through molten aluminium oxide for 36 hrs.Calculate the amount of aluminium deposited in kg.(Al = 27, IF =...(Solved)
A current of 25 amps was passed through molten aluminium oxide for 36 hrs.Calculate the amount of aluminium deposited in kg.(Al = 27, IF = 96500C)
Date posted: May 17, 2019.
- Aluminium is extracted from its ore by electrolysis method. The current required in the
process is 4,000 amperes. Study the diagram and answer the questions that...(Solved)
Aluminium is extracted from its ore by electrolysis method. The current required in the
process is 4,000 amperes. Study the diagram and answer the questions that follow.
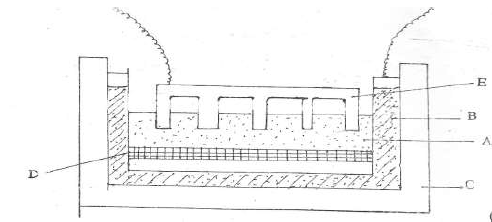 (a) Name:
(i) Electrolyte A
(ii) Substance D
(iii) Electrode E
(b) Name the material from which the electrodes are made.
(c ) (i) Write the equation that produces aluminium metal.
(ii) Explain why E has to be replaced from time to time.
(iii) Why is cryolite added to the electrolyte before the process of electrolysis ?
(a) Name:
(i) Electrolyte A
(ii) Substance D
(iii) Electrode E
(b) Name the material from which the electrodes are made.
(c ) (i) Write the equation that produces aluminium metal.
(ii) Explain why E has to be replaced from time to time.
(iii) Why is cryolite added to the electrolyte before the process of electrolysis ?
Date posted: May 17, 2019.
- In an experiment to determine the molar heat of neutralization of hydrochloric acid
with sodium hydroride, students of Furaha Secondary school reacted 100cm3 of 1M
hydrochloric acid...(Solved)
In an experiment to determine the molar heat of neutralization of hydrochloric acid
with sodium hydroride, students of Furaha Secondary school reacted 100cm3 of 1M
hydrochloric acid with 50cm3 of 2M sodium hydroxide solution. They obtained the following results.
Initial temperature of acid = 25.00C
Initial temperature of base = 25.00C
Highest temperature reached
With the acid – alkali mixture = 34.00C
(a) Write an ionic equation for the neutralization reaction between hydrochloric acid
and sodium hydroxide.
(b ) Calculate :
(i) The change in temperature.
(ii) The amount of heat produced during the reaction.
( Specific heat capacity of solution = 4.2 kJkg-1k-1 )
(iii) The molar heat of neutralization of sodium hydroxide.
(c) Write the thermochemical equation for the reaction.
(d) Draw an energy level diagram for the reaction.
Date posted: May 17, 2019.
- Define the term molar heat of neutralization.(Solved)
Define the term molar heat of neutralization.
Date posted: May 17, 2019.
- State two uses of carbon (IV) oxide.(Solved)
State two uses of carbon (IV) oxide.
Date posted: May 17, 2019.
- State one environmental effect that excess carbon (IV) oxide in the air causes.(Solved)
State one environmental effect that excess carbon (IV) oxide in the air causes.
Date posted: May 17, 2019.
- A piece of marble chip ( calcium carbonate) is put in a beaker containing excess of dilute hydrochloric
acid which is placed on a reading balance....(Solved)
A piece of marble chip ( calcium carbonate) is put in a beaker containing excess of dilute hydrochloric
acid which is placed on a reading balance. The mass of the beaker and its contents is recorded every
two minutes, as shown in the table.
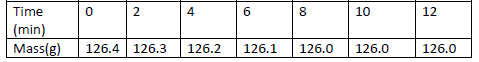 (a) Why is there a continuous loss of mass of the reaction mixture.
(b) Write an equation for the reaction taking place.
(c ) State two different ways by which the reaction could have been made more rapid.
(d) Why does the mass remain constant after 8 minutes.
(e) State the observations that would be made if a few drops of silver nitrate solution
Was added to 1cm3 of the resulting solution followed by ammonia solution.
(a) Why is there a continuous loss of mass of the reaction mixture.
(b) Write an equation for the reaction taking place.
(c ) State two different ways by which the reaction could have been made more rapid.
(d) Why does the mass remain constant after 8 minutes.
(e) State the observations that would be made if a few drops of silver nitrate solution
Was added to 1cm3 of the resulting solution followed by ammonia solution.
Date posted: May 17, 2019.
- The standard electrode potentials for some half cells are given below.(Solved)
The standard electrode potentials for some half cells are given below.
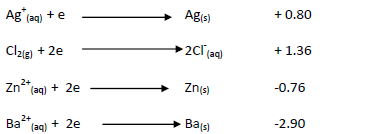 i)Arrange the metals in order of reactivity.
ii)Calculate the E.M.F of the cell shown below.
i)Arrange the metals in order of reactivity.
ii)Calculate the E.M.F of the cell shown below.
 iii) What would happen if a cell with chlorine and zinc ions, the anode was made
of zinc. Explain your answer.
iii) What would happen if a cell with chlorine and zinc ions, the anode was made
of zinc. Explain your answer.
Date posted: May 17, 2019.
- The set-up below represents electrolysis of dilute sulphuric (VI) acid.(Solved)
The set-up below represents electrolysis of dilute sulphuric (VI) acid.
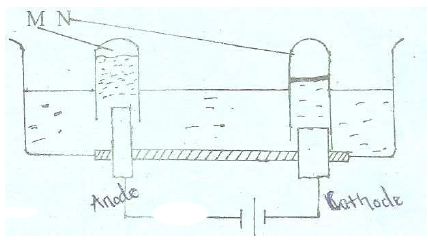 (a) Identify gases M and N
(b) Write an ionic equation for the production of gas M.
(c ) At what electrode does reduction take place. Explain your answer.
(d) State the most suitable electrodes that can be used in this experiment.
Explain your answer.
(a) Identify gases M and N
(b) Write an ionic equation for the production of gas M.
(c ) At what electrode does reduction take place. Explain your answer.
(d) State the most suitable electrodes that can be used in this experiment.
Explain your answer.
Date posted: May 17, 2019.
- Give the IUPAC names of the following compounds.(Solved)
Give the IUPAC names of the following compounds.

Date posted: May 17, 2019.
- The scheme below shows various reactions starting with ammonia. Study it and
answer the questions that follow.(Solved)
The scheme below shows various reactions starting with ammonia. Study it and
answer the questions that follow.
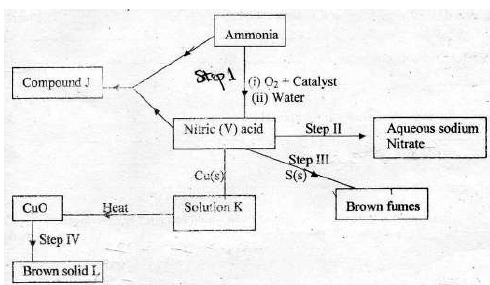 (i) List the raw materials used in the manufacture of ammonia.
(ii) What catalyst is used in step I ?
(iii) Write an equation for the reaction that occurs between ammonia and oxygen in
presence of the catalyst.
(iv) Identify the process in step II
(v) Using an appropriate equation, explain how the reaction in step III occurs ?
(vi) What should be added to solution K to form solid L ?
(vii) (a) (i) Write the formula of compound J.
(ii) Calculate the mass of compound J that would contain 14g of nitrogen
( H = 1, N = 14, O = 16 )
(b) State two advantages of ammonium phosphate over ammonium nitrate.
(i) List the raw materials used in the manufacture of ammonia.
(ii) What catalyst is used in step I ?
(iii) Write an equation for the reaction that occurs between ammonia and oxygen in
presence of the catalyst.
(iv) Identify the process in step II
(v) Using an appropriate equation, explain how the reaction in step III occurs ?
(vi) What should be added to solution K to form solid L ?
(vii) (a) (i) Write the formula of compound J.
(ii) Calculate the mass of compound J that would contain 14g of nitrogen
( H = 1, N = 14, O = 16 )
(b) State two advantages of ammonium phosphate over ammonium nitrate.
Date posted: May 17, 2019.
- The grid given below represents part of the periodic table. Study it and answer the questions
that follow. The letters are not the actual symbols of...(Solved)
The grid given below represents part of the periodic table. Study it and answer the questions
that follow. The letters are not the actual symbols of the elements.
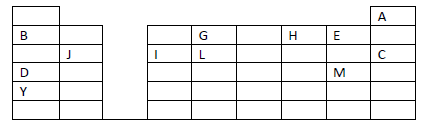 (i) What name is given to the family of elements to which A and C belong?
(ii) Write the chemical formula of the sulphate of element D.
(iii ) Which letter represents the most reactive
(a) Metal
(b) Non-metal
(iv) Name the bond formed when B and H react. Explain your answer.
(v) Select one element that belong to period 4.
(vi) Ionic radius of element E is bigger than the atomic radius. Explain.
(vii) The electron configuration of a divalent anion of element N is 2.8.8. Induce the
position of element N on the periodic table drawn above.
(viii) The oxide of G has a lower melting point than the oxide of L. Explain.
(ix) How do the atomic radii of I and C compare. Explain.
(x) Explain the trend in the 1st ionization energies of the elements J, I and L.
(i) What name is given to the family of elements to which A and C belong?
(ii) Write the chemical formula of the sulphate of element D.
(iii ) Which letter represents the most reactive
(a) Metal
(b) Non-metal
(iv) Name the bond formed when B and H react. Explain your answer.
(v) Select one element that belong to period 4.
(vi) Ionic radius of element E is bigger than the atomic radius. Explain.
(vii) The electron configuration of a divalent anion of element N is 2.8.8. Induce the
position of element N on the periodic table drawn above.
(viii) The oxide of G has a lower melting point than the oxide of L. Explain.
(ix) How do the atomic radii of I and C compare. Explain.
(x) Explain the trend in the 1st ionization energies of the elements J, I and L.
Date posted: May 17, 2019.
- Below are the bond dissociation energies of some elements.(Solved)
Below are the bond dissociation energies of some elements.
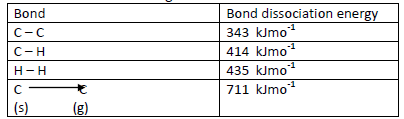 Use this information to calculate the heat of reaction for:-
Use this information to calculate the heat of reaction for:-
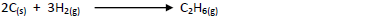
Date posted: May 17, 2019.
- Study the arrangement below and answer the question that follows.
(Solved)
Study the arrangement below and answer the question that follows.
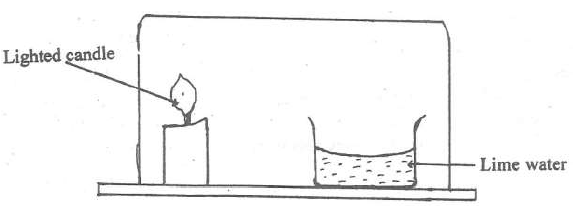 Explain what will be observed after some time.
Explain what will be observed after some time.
Date posted: May 17, 2019.
- The diagram below outlines industrial preparation of bleaching powder.(Solved)
The diagram below outlines industrial preparation of bleaching powder.
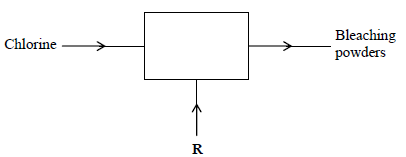 (i) Give the chemical name of bleaching powder
(ii) Identify substance R
(iii) Explain why water in which bleaching powder has been added needs a lot of soap
during washing.
(i) Give the chemical name of bleaching powder
(ii) Identify substance R
(iii) Explain why water in which bleaching powder has been added needs a lot of soap
during washing.
Date posted: May 17, 2019.
- The reaction of water and calcium gave gas Q collected as in the diagram below(Solved)
The reaction of water and calcium gave gas Q collected as in the diagram below
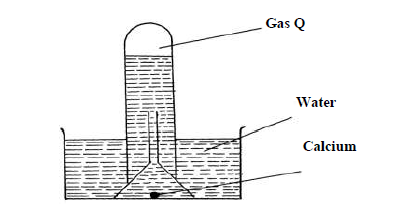 (i) Identify gas Q
(ii) Explain why the solution left after the reaction is a weak base.
(i) Identify gas Q
(ii) Explain why the solution left after the reaction is a weak base.
Date posted: May 17, 2019.
- On heating Ammonium Chloride two gas P and J were evolved G turned moist litmus
paper red and J turned Moist litmus paper blue. On cooling...(Solved)
On heating Ammonium Chloride two gas P and J were evolved G turned moist litmus
paper red and J turned Moist litmus paper blue. On cooling , the two gases
recombined to form a white solid
a) Identify P and J
b) What property of Ammonium Chloride is shown in this experiment?
Date posted: May 17, 2019.
- A solution of hydrogen chloride gases in water reacts with Zinc carbonate, but a solution of hydrogen chloride in methylbenzene does not . Explain(Solved)
A solution of hydrogen chloride gases in water reacts with Zinc carbonate, but a solution of hydrogen chloride in methylbenzene does not. Explain
Date posted: May 17, 2019.
- Dry hydrogen chloride gas was passed through heated iron wire as shown in the diagram
below(Solved)
Dry hydrogen chloride gas was passed through heated iron wire as shown in the diagram
below
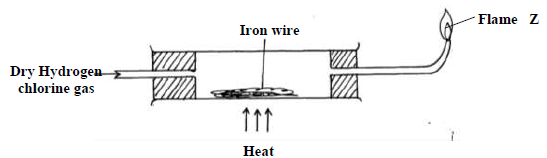 a) (i) How can the identity of the substance burning with flame Z be confirmed.
(ii) What is observed in combustion tube during the experiment?
(iii) Write the equation for the reaction taking place in the combustion tube.
(iv) Chlorine gas was passed over the product obtained in the combustion tube to give
another product Q
a) State one precaution that should be taken. Explain
b) Identify product Q
c) The total mass of product Q formed was found to be 5.3g. Calculate the volume of
chlorine gas used.
(Cl = 35.5, Fe= 56, Molar gas volume at room temperature = 2400cm3)
a) (i) How can the identity of the substance burning with flame Z be confirmed.
(ii) What is observed in combustion tube during the experiment?
(iii) Write the equation for the reaction taking place in the combustion tube.
(iv) Chlorine gas was passed over the product obtained in the combustion tube to give
another product Q
a) State one precaution that should be taken. Explain
b) Identify product Q
c) The total mass of product Q formed was found to be 5.3g. Calculate the volume of
chlorine gas used.
(Cl = 35.5, Fe= 56, Molar gas volume at room temperature = 2400cm3)
Date posted: May 17, 2019.
- The flow chart below outlines some of the process involved during extraction of copper.(Solved)
The flow chart below outlines some of the process involved during extraction of copper.
 (i) Write the formula of copper pyrite.
(ii) Name liquid T
(iii) Write equations for the reactions taking place in the 2nd roasting furnace.
(iv) Identify substance B and write equation for the reaction that take place in the smelting furnace.
(v) State the purpose of substance F.
(i) Write the formula of copper pyrite.
(ii) Name liquid T
(iii) Write equations for the reactions taking place in the 2nd roasting furnace.
(iv) Identify substance B and write equation for the reaction that take place in the smelting furnace.
(v) State the purpose of substance F.
Date posted: May 17, 2019.
- Study the scheme below and answer the questions that follow.(Solved)
Study the scheme below and answer the questions that follow.
 (i) Identify the product B.
(ii) Name the compounds C and E.
(iii) State the conditions for step 1
(iv) Write the equation for the reaction leading tot the formation of methane.
(v) State two industrial uses of methane.
(vi) Identify the reagent D
(i) Identify the product B.
(ii) Name the compounds C and E.
(iii) State the conditions for step 1
(iv) Write the equation for the reaction leading tot the formation of methane.
(v) State two industrial uses of methane.
(vi) Identify the reagent D
Date posted: May 17, 2019.
- Name the following compounds.(Solved)
Name the following compounds.
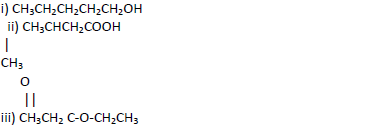
Date posted: May 17, 2019.
- The graph below was obtained from an experiment used to investigate the reaction
between Zinc granules and 2M hydrochloric acid.(Solved)
The graph below was obtained from an experiment used to investigate the reaction
between Zinc granules and 2M hydrochloric acid.
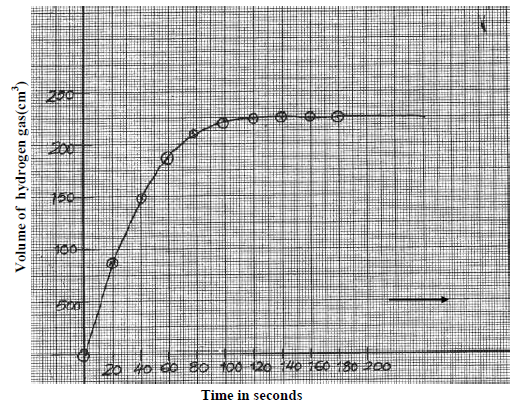 a) Calculate the rate of reaction when t = 60seconds.
b) Suggest how the rate of the above reaction can be reduced so that it can be studied more closely at the same temperature.
a) Calculate the rate of reaction when t = 60seconds.
b) Suggest how the rate of the above reaction can be reduced so that it can be studied more closely at the same temperature.
Date posted: May 17, 2019.
- Describe two observable characteristics of a luminous flame(Solved)
Describe two observable characteristics of a luminous flame.
Date posted: May 17, 2019.
- Write a chemical equation for the combustion of laboratory gas , when the
Bunsen burner produces a non-luminous flame.(Solved)
Write a chemical equation for the combustion of laboratory gas , when the
Bunsen burner produces a non-luminous flame.
Date posted: May 17, 2019.
- Explain why during the extraction of metals, copper can be extracted by the electrolysis of
copper (II) sulphate solution, while aluminium chloride cannot be extracted by...(Solved)
Explain why during the extraction of metals, copper can be extracted by the electrolysis of
copper (II) sulphate solution, while aluminium chloride cannot be extracted by electrolysis of
aluminium sulphate solution.
Date posted: May 17, 2019.
- Study the following equilibrium equation and answer the questions that follows.(Solved)
Study the following equilibrium equation and answer the questions that follows.
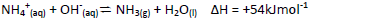 Explain how ammonia gas can be prepared in the laboratory.
Explain how ammonia gas can be prepared in the laboratory.
Date posted: May 17, 2019.
- The following are standard electrode potentials of some half-cell reactions. Use the data to
answer the questions that follow.(Solved)
The following are standard electrode potentials of some half-cell reactions. Use the data to
answer the questions that follow.
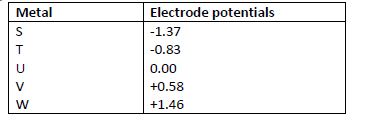 i) Suggest the identify of element U.
ii) Draw a labeled diagram of an electrochemical cell that would produce the largest e.m.f.
i) Suggest the identify of element U.
ii) Draw a labeled diagram of an electrochemical cell that would produce the largest e.m.f.
Date posted: May 17, 2019.
- The following diagram represents a set-up used to investigate conditions necessary for rusting of iron.(Solved)
The following diagram represents a set-up used to investigate conditions necessary for rusting of iron.
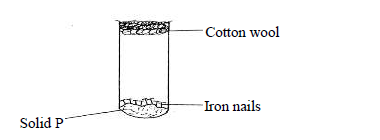 After several days it was found that the nails did not rust. Identify solid P.
After several days it was found that the nails did not rust. Identify solid P.
Date posted: May 17, 2019.